Poster Presentation The Joint Annual Scientific Meetings of the Endocrine Society of Australia and the Society for Reproductive Biology 2017
Close contact of an endocrinological kind (#259)
Introduction:
Testosterone transdermal gel preparations commonly prescribed for androgen deficiency in men carry an important safety concern of transfer of testosterone to women and children with subsequent development of hyperandrogenism (1, 2).
We present a paediatric case of hyperandrogenism following transfer of topical testosterone gel.
All analysis was performed using a high-sensitivity liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for simultaneous measurement of androgen steroids using a liquid-liquid extraction method.
Case:
A 7 month-old female infant presented with rapid development of pubic hair over labia major from 6th month of age. Examination findings included clitoromegaly, scrotalised labia major, pubic hair (Tanner 3 stage), prominent dark nipples and palpable breast tissue bilaterally. At presentation serum testosterone was 12 nmol/L; the suspected source via skin-to-skin transfer from the father. He had been undergoing androgen replacement with application of bioidentical androgen gel reportedly containing 15% Testosterone, 4% DHEAS and 0.5% Progesterone to the upper arm. Serum testosterone decreased upon cessation of exogenous exposure but has yet to normalise after 8 weeks. Infant remains under surveillance for development of central precocious puberty as androgen levels decline.
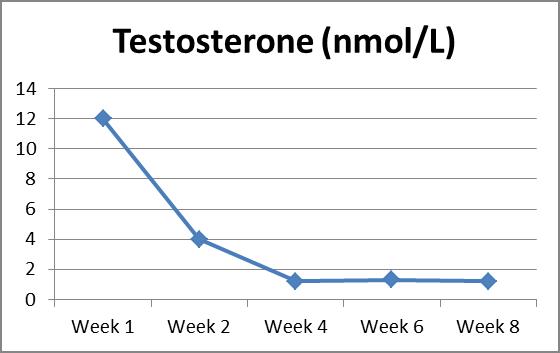
Discussion: Conversion of testosterone to the more potent androgen receptor agonist and highly active metabolite dihydrotestosterone, a process catalysed by 5-alpha reductase, is likely to be responsible for virilisation of this infant girl (3). This may potentially trigger precious puberty and review of case reports in the literature will be discussed.
Conclusion: We are postulating that the difference in fat amount and distribution in infant may explain slow decline in testosterone level following exogenous exposure.