Oral Presentation The Joint Annual Scientific Meetings of the Endocrine Society of Australia and the Society for Reproductive Biology 2017
Effects of triiodothyronine on energy expenditure and cardiovascular risk in two patients with a mutation in thyroid hormone receptor β (#153)
Background: Triiodothyronine (T3), the most active thyroid hormone, stimulates energy expenditure, but is not a viable weight loss therapy due to potential adverse cardiovascular effects. T3 binds to two receptors: TRα predominates in cardiac and skeletal muscle and TRβ in the central nervous system. Mutations in TRβ provide an opportunity to assess the effect of selective TRβ receptor stimulation as a potential therapeutic target for weight loss, while avoiding adverse cardiovascular effects.
Methods: Six healthy female controls (age 36±3 years, fT3=4.3±0.2 pmol/L) and two female patients with mutations in TRβ (age 25 and 40 years, fT3=7.8 and 10.0 pmol/L) were studied before and after two days of T3 (100 µg/day). Resting energy expenditure (REE) and diet-induced thermogenesis (DIT) were assessed by indirect calorimetry performed before and after a mixed meal. Pulse, blood pressure (BP) and low-density lipoprotein cholesterol (LDL-C) were measured and brown adipose tissue (BAT) activity was assessed by infrared thermography.
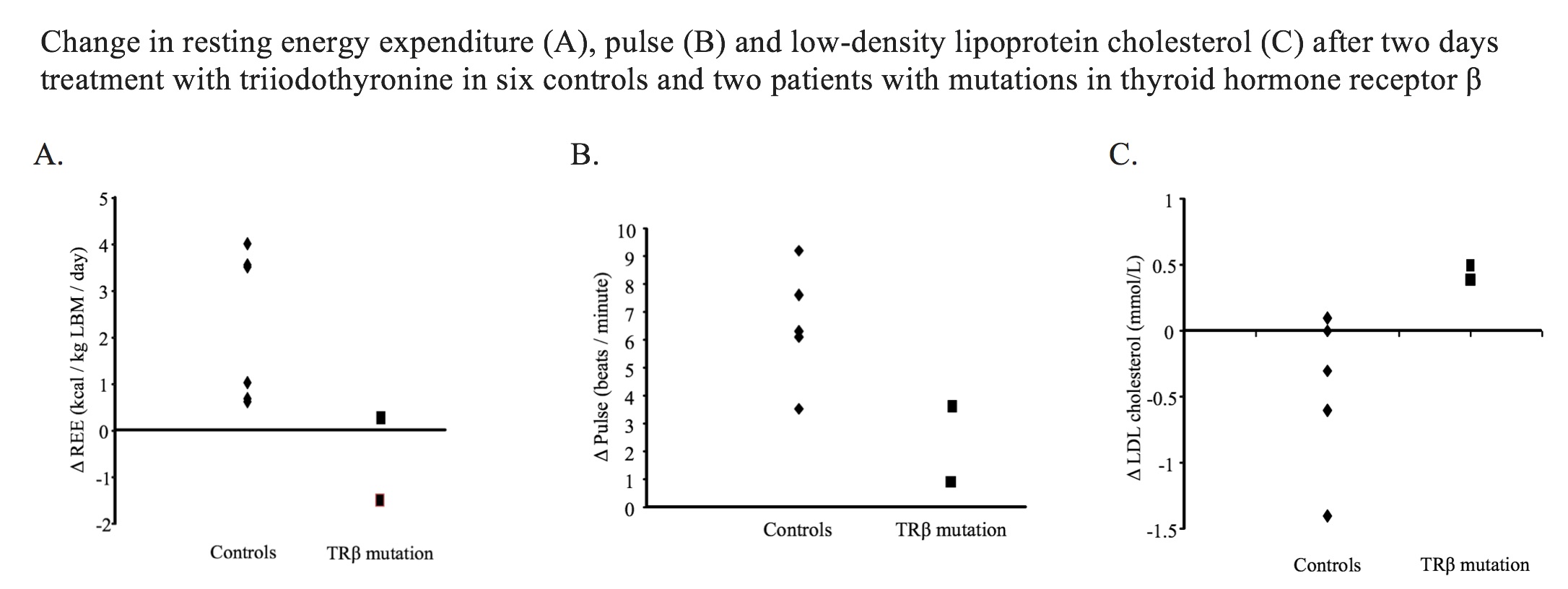
Results: Following T3 treatment, mean ΔfT3 was +15.5±1.6 pmol/L in controls and +15.3±3.3 pmol/L in patients with TRβ mutations. In controls, T3 increased REE (Δ+2.3±0.7 kcal/kg LBM/day, p=0.02) and pulse (Δ+ 6.5±0.8 bpm, p<0.001) and tended to reduce LDL-C (Δ-0.5±0.2 mmol/L, p=0.09). T3 did not affect DIT (p=0.98), BAT activity (p=0.18) or systolic BP (p=0.81). Patients with TRβ mutations had higher REE and pulse, but not LDL-C, at baseline, but an attenuated change in REE, pulse and LDL-C after T3 (Figure). DIT, BAT activity and BP did not differ between groups.
Conclusions: T3 effects on REE and heart rate are mediated by both TRα and TRβ, while change in LDL-C is via TRβ. Selective TRβ stimulation will likely increase REE with consequent weight loss and reduce LDL-C. However, although the effect on heart rate is predicted to be less than T3, an increase in pulse is expected.